close
JP Kagu 日系高型質感電視收納櫃
JP Kagu 日系高型質感電視收納櫃
JP Kagu 日系高型質感電視收納櫃

想購買請點我
商品訊息功能:
商品訊息描述:
JP Kagu 日系高型質感電視收納櫃
此商品為組裝品








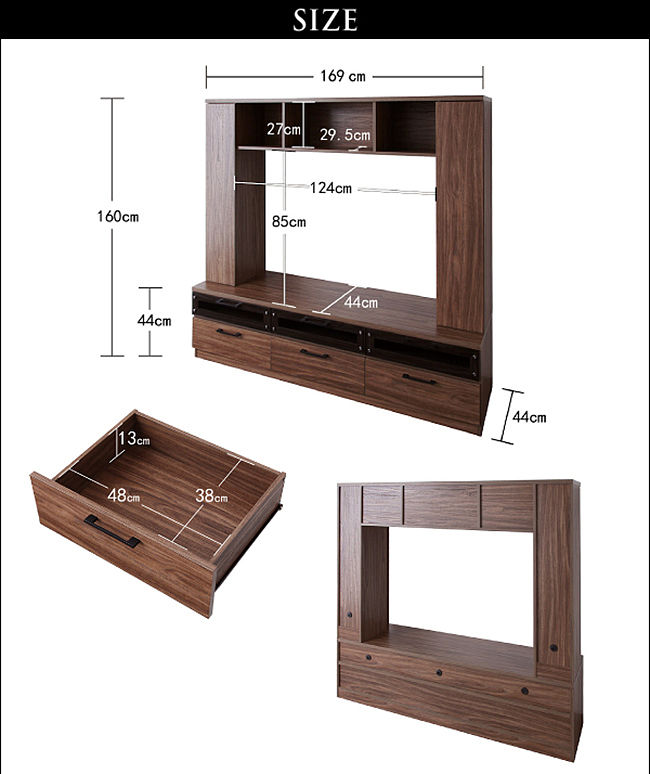



商品訊息簡述:
JP Kagu 日系高型質感電視收納櫃

想購買請點我
2017-03-1003:00
INVESTIGATION: Minister of Health and Welfare Chen Shih-chung said authorities are trying to identify those involved in the production and distribution of the counterfeits
By Wu Liang-yi, Lee Hsin-fang and Jonathan Chin / Staff reporters, with staff writer
Counterfeit batches of Crestor brand lipid-lowering drugs contained ingredients from Chinese pharmaceutical producers, Minister of Health and Welfare Chen Shih-chung (陳時中) said yesterday after being subjected to a barrage of questions from a lawmaker.
A recall was issued on Tuesday for all of AstraZeneca’s Crestor tablets following a discovery last week that two batches of the drug prescribed to 570,000 National Health Insurance patients were counterfeits made from atorvastatin, a cheaper drug whose patent had expired.
The Ministry of Health and Welfare did not release any information regarding the quantity, distribution or origin of the fake drugs until yesterday, when Democratic Progressive Party Legislator Liu Chien-kuo (劉建國) grilled Chen on the issue during a legislative session.
Following Liu’s questions, Chen said that the fake drugs’ ingredients were mailed from China to Taiwan, but the identity of many individuals involved in their production and distribution remained unknown to authorities.
The ministry is considering sending investigators to China, Chen said, adding that judicial authorities have taken charge of the investigation.
Liu said that the majority of the imported products seized by the Food and Drug Administration at customs offices for being counterfeits or failing to meet safety standards originated in China.
Separately yesterday, Premier Lin Chuan (林全) pledged to comprehensively overhaul the nation’s drug safety standards during a meeting he chaired at the Executive Yuan.
“This incident is a test for our entire medical safety system and especially for the ministry’s agencies responsible for protecting the nation’s drug safety, as it will show whether their work has won the public’s trust,” Lin said. “We must use this opportunity to completely re-evaluate our performance.”
The primary suspect involved in counterfeiting the drug has been apprehended, Lin said, acknowledging the efforts of police, prosecutors and the ministry to hold the suspect accountable.
The government has requested the assistance of the pharmaceutical sector in recalling and replacing the affected products, he added.
The ministry must ensure the complete recovery and destruction of the fake drugs, and it must make information available to avoid an unnecessary panic, Lin said, adding that an inspection of all commonly prescribed and high-value pharmaceuticals would begin immediately.
Lin said the Cabinet would at the earliest opportunity review existing procedures, including those for dealing with fake drugs; tracking the production and distribution of medicines; quality control regulations; hospital inventory inspections; the mechanism for reporting fake drugs; and methods for their disposal.
The government must protect the public’s access to safe medicines that are essential for public health, uphold the nation’s reputation for effective regulation of medical products and restore public confidence in the government, he added.
新聞來源:TAIPEI TIMES404F140EB30ADF16
JP Kagu 日系高型質感電視收納櫃
JP Kagu 日系高型質感電視收納櫃

想購買請點我
商品訊息功能:
商品訊息描述:
JP Kagu 日系高型質感電視收納櫃
此商品為組裝品













商品訊息簡述:
JP Kagu 日系高型質感電視收納櫃

想購買請點我
2017-03-1003:00
INVESTIGATION: Minister of Health and Welfare Chen Shih-chung said authorities are trying to identify those involved in the production and distribution of the counterfeits
By Wu Liang-yi, Lee Hsin-fang and Jonathan Chin / Staff reporters, with staff writer
Counterfeit batches of Crestor brand lipid-lowering drugs contained ingredients from Chinese pharmaceutical producers, Minister of Health and Welfare Chen Shih-chung (陳時中) said yesterday after being subjected to a barrage of questions from a lawmaker.
A recall was issued on Tuesday for all of AstraZeneca’s Crestor tablets following a discovery last week that two batches of the drug prescribed to 570,000 National Health Insurance patients were counterfeits made from atorvastatin, a cheaper drug whose patent had expired.
The Ministry of Health and Welfare did not release any information regarding the quantity, distribution or origin of the fake drugs until yesterday, when Democratic Progressive Party Legislator Liu Chien-kuo (劉建國) grilled Chen on the issue during a legislative session.
Following Liu’s questions, Chen said that the fake drugs’ ingredients were mailed from China to Taiwan, but the identity of many individuals involved in their production and distribution remained unknown to authorities.
The ministry is considering sending investigators to China, Chen said, adding that judicial authorities have taken charge of the investigation.
Liu said that the majority of the imported products seized by the Food and Drug Administration at customs offices for being counterfeits or failing to meet safety standards originated in China.
Separately yesterday, Premier Lin Chuan (林全) pledged to comprehensively overhaul the nation’s drug safety standards during a meeting he chaired at the Executive Yuan.
“This incident is a test for our entire medical safety system and especially for the ministry’s agencies responsible for protecting the nation’s drug safety, as it will show whether their work has won the public’s trust,” Lin said. “We must use this opportunity to completely re-evaluate our performance.”
The primary suspect involved in counterfeiting the drug has been apprehended, Lin said, acknowledging the efforts of police, prosecutors and the ministry to hold the suspect accountable.
The government has requested the assistance of the pharmaceutical sector in recalling and replacing the affected products, he added.
The ministry must ensure the complete recovery and destruction of the fake drugs, and it must make information available to avoid an unnecessary panic, Lin said, adding that an inspection of all commonly prescribed and high-value pharmaceuticals would begin immediately.
Lin said the Cabinet would at the earliest opportunity review existing procedures, including those for dealing with fake drugs; tracking the production and distribution of medicines; quality control regulations; hospital inventory inspections; the mechanism for reporting fake drugs; and methods for their disposal.
The government must protect the public’s access to safe medicines that are essential for public health, uphold the nation’s reputation for effective regulation of medical products and restore public confidence in the government, he added.
新聞來源:TAIPEI TIMES404F140EB30ADF16
文章標籤
全站熱搜


 留言列表
留言列表


